Interleukin-2 Inhibitors Clinical Trial Pipeline Accelerates as 20+ Pharma Companies Rigorously Develop Drugs for Market Entry | DelveInsight
Interleukin-2 (IL-2) inhibitors are immunosuppressive agents that block the activity of IL-2, a cytokine involved in the activation and proliferation of T cells. The growing prevalence of autoimmune diseases and organ transplant procedures is driving demand for IL-2 inhibitors due to their critical role in preventing immune rejection and inflammation.
New York, USA, July 10, 2025 (GLOBE NEWSWIRE) -- Interleukin-2 Inhibitors Clinical Trial Pipeline Accelerates as 20+ Pharma Companies Rigorously Develop Drugs for Market Entry | DelveInsight
Interleukin-2 (IL-2) inhibitors are immunosuppressive agents that block the activity of IL-2, a cytokine involved in the activation and proliferation of T cells. The growing prevalence of autoimmune diseases and organ transplant procedures is driving demand for IL-2 inhibitors due to their critical role in preventing immune rejection and inflammation.
DelveInsight’s 'Interleukin-2 Inhibitors Pipeline Insight 2025' report provides comprehensive global coverage of pipeline interleukin-2 inhibitors in various stages of clinical development, major pharmaceutical companies are working to advance the pipeline space and future growth potential of the interleukin-2 inhibitors pipeline domain.
Key Takeaways from the Interleukin-2 Inhibitors Pipeline Report
- DelveInsight’s interleukin-2 inhibitors pipeline report depicts a robust space with 20+ active players working to develop 22+ pipeline interleukin-2 inhibitors.
- Key interleukin-2 inhibitor companies, such as Corvus Pharmaceuticals, Philogen, Nektar, Cue Biopharma, ILTOO Pharma, Innovent, Regeneron Pharmaceuticals, XEME Biopharma, AstraZeneca, GI Innovation, Merck, Ascendis Pharma, Sanofi, AbbVie, Equillium, Anaveon, Aulos Bioscience, Selecxine, ProBio, Hoffmann-La Roche, TILT Biotherapeutics, and others, are evaluating new interleukin-2 inhibitor drugs to improve the treatment landscape.
- Promising pipeline interleukin-2 inhibitors such as Soquelitinib (CPI-818), Darleukin (L19IL2), MK-6194, Rezpegaldesleukin (REZPEG, NKTR-358, LY3471851), CUE-101, ILT-101, IBI363, REGN7257, Oncoquest-L Vaccine, GI-101/GI-101A, Oncoquest-CLL Vaccine, SAR444336, TransCon IL-2 β/γ, Pegenzileukin/THOR-707 (SAR444245), EQ 101, ANV419, AU-007, SLC-3010, Eciskafusp alfa (PD1-IL-2v, RG6279), TILT-123, and others are under different phases of interleukin-2 inhibitors clinical trials.
- In March 2025, The development of nemvaleukin in platinum-resistant ovarian cancer will be discontinued based on interim OS data from the Phase III ARTISTRY-7 trial which failed to show a statistically significant improvement in overall survival (OS) with nemvaleukin alfa (ALKS 4230) in combination with pembrolizumab (Keytruda) vs investigator’s choice chemotherapy in patients with platinum-resistant ovarian cancer, the study will not continue to final analysis.
- In February 2025, The FDA had granted a fast track designation to rezpegaldesleukin for treating patients aged 12 years and older with moderate to severe atopic dermatitis that topical therapies have not adequately controlled.
- In November 2024, Synthekine presented the preclinical data for CD19 CAR-T (SYNCAR-001) empowered by orthogonal IL-2 (STK-009) without lymphodepletion at the American College of Rheumatology (ACR) convergence.
- In October 2024, Medicenna Therapeutics, announced that new data from two of its preclinical programs, MDNA209 and MDNA113 are preclinical assets based on the MDNA109 platform also used to develop MDNA11, a long-acting IL-2 Super-agonist, currently being evaluated in the Phase I/II ABILITY-1 clinical trial for the treatment of solid tumors.
- In September 2024, SYNCAR-001 + STK-009 received Fast Track Designation (FTD) from the US FDA for patients with severe, refractory systemic lupus erythematosus (SLE), without the use of lymphodepletion.
Request a sample and discover the recent advances in interleukin-2 inhibitor drugs @ Interleukin-2 Inhibitors Pipeline Report
The interleukin-2 inhibitors pipeline report provides detailed profiles of pipeline assets, a comparative analysis of clinical and non-clinical stage interleukin-2 inhibitors drugs, inactive and dormant assets, a comprehensive assessment of driving and restraining factors, and an assessment of opportunities and risks in the interleukin-2 inhibitors clinical trial landscape.
Interleukin-2 Inhibitors Overview
Interleukin-2 (IL-2) inhibitors are a group of immunosuppressive drugs aimed at blocking the function of IL-2, a key cytokine involved in immune system regulation. IL-2 is mainly secreted by activated T cells and is vital for the growth, specialization, and survival of various T-cell types, including both regulatory (Tregs) and effector T cells. It exerts its effects through the IL-2 receptor (IL-2R), which is made up of three parts: IL-2Rα (CD25), IL-2Rβ (CD122), and IL-2Rγ (CD132). Binding of IL-2 to this receptor triggers multiple intracellular pathways, such as JAK-STAT, PI3K, and MAPK, which influence immune cell behavior.
IL-2 inhibitors come in three primary forms: monoclonal antibodies, small molecules, and fusion proteins. Monoclonal antibodies are large, specific proteins that attach to particular IL-2 receptor components, preventing IL-2 from activating downstream signaling. Their high specificity allows for targeted immune modulation with minimal impact on other cytokine networks.
Clinically, IL-2 inhibitors are significant due to their ability to precisely control immune responses. By interfering with IL-2 signaling, they can suppress harmful immune reactions, such as inflammation or tissue damage in autoimmune diseases or organ transplantation. In other cases, modulating this pathway can help fine-tune the immune response, either dampening or enhancing specific T-cell populations. This precise control makes IL-2 inhibitors valuable therapeutic agents for improving disease outcomes and personalized immune management.
Find out more about interleukin-2 inhibitors drugs @ Interleukin-2 Inhibitors Analysis
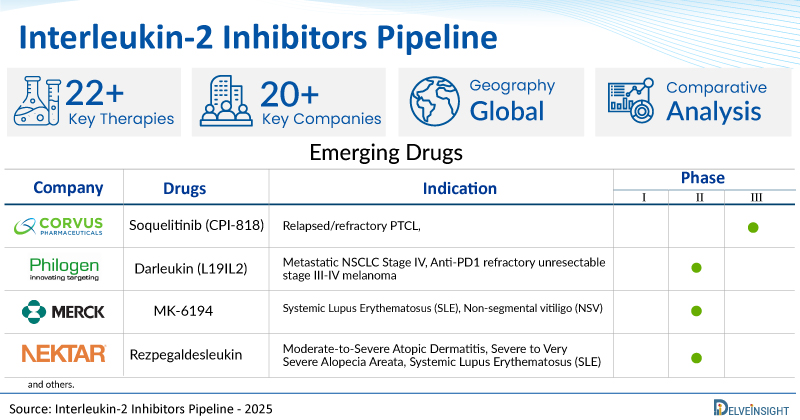
A snapshot of the Pipeline Interleukin-2 Inhibitors Drugs mentioned in the report:
| Company | Drugs | Phase | Indication | RoA |
| Corvus Pharmaceuticals | Soquelitinib (CPI-818) | III | Relapsed/refractory PTCL, | Oral |
| Philogen | Darleukin (L19IL2) | II | Metastatic NSCLC Stage IV, Anti-PD1 refractory unresectable stage III-IV melanoma | Intravenous |
| Merck | MK-6194 | II | Systemic Lupus Erythematosus (SLE), Non-segmental vitiligo (NSV) | Subcutaneous |
| Nektar Therapeutics | Rezpegaldesleukin (REZPEG/NKTR-358) | II | Moderate-to-Severe Atopic Dermatitis, Severe to Very Severe Alopecia Areata, Systemic Lupus Erythematosus (SLE) | Subcutaneous |
| ILTOO Pharma | ILT-101 | II | Type 1 diabetes, Newly diagnosed ALS, Bipolar disorder, Moderate to severe SLE, Acute respiratory distress syndrome related to COVID-19, Autoimmune and inflammatory diseases | Subcutaneous |
| Cue Biopharma | CUE-101 | II | Locally advanced HPV16+ oropharyngeal squamous-cell carcinoma (OPSCC) | Intravenous |
| Equillium | EQ 101 | II | LGL Leukemia or Refractory CTCL, Moderate to Severe Alopecia Areata | Intravenous |
| XEME Biopharma | Oncoquest-L vaccine | II | Stage III or IV asymptomatic, non-bulky follicular lymphoma | Subcutaneous |
| Anaveon | ANV419 | II | Relapsed/Refractory Advanced Solid Tumors | Intravenous |
| Regeneron Pharmaceuticals | REGN7257 | II | Severe Aplastic Anemia | Intravenous |
Learn more about the emerging interleukin-2 inhibitors @ Interleukin-2 Inhibitors Clinical Trials
Interleukin-2 Inhibitors Therapeutics Assessment
The interleukin-2 inhibitors pipeline report proffers an integral view of the emerging interleukin-2 inhibitors segmented by stage, product type, molecule type, and route of administration.
Scope of the Interleukin-2 Inhibitors Pipeline Report
- Coverage: Global
- Therapeutic Assessment By Product Type: Mono, Combination, Mono/Combination
- Therapeutic Assessment By Clinical Stages: Discovery, Pre-clinical, Phase I, Phase II, Phase III
- Therapeutics Assessment By Route of Administration: Intra-articular, Intraocular, Intrathecal, Intravenous, Oral, Parenteral, Subcutaneous, Topical, Transdermal
- Therapeutics Assessment By Molecule Type: Oligonucleotide, Peptide, Small molecule
- Key Interleukin-2 Inhibitors Companies: Corvus Pharmaceuticals, Philogen, Nektar, Cue Biopharma, ILTOO Pharma, Innovent, Regeneron Pharmaceuticals, XEME Biopharma, AstraZeneca, GI Innovation, Merck, Ascendis Pharma, Sanofi, AbbVie, Equillium, Anaveon, Aulos Bioscience, Selecxine, ProBio, Hoffmann-La Roche, TILT Biotherapeutics, and others
- Key Pipeline Interleukin-2 Inhibitors: Soquelitinib (CPI-818), Darleukin (L19IL2), MK-6194, Rezpegaldesleukin (REZPEG, NKTR-358, LY3471851), CUE-101, ILT-101, IBI363, REGN7257, Oncoquest-L Vaccine, GI-101/GI-101A, Oncoquest-CLL Vaccine, SAR444336, TransCon IL-2 β/γ, Pegenzileukin/THOR-707 (SAR444245), EQ 101, ANV419, AU-007, SLC-3010, Eciskafusp alfa (PD1-IL-2v, RG6279), TILT-123, and others
Dive deep into rich insights for new interleukin-2 inhibitors, visit @ Interleukin-2 Inhibitors Drugs
Table of Contents
| 1. | Interleukin-2 Inhibitors Pipeline Report Introduction |
| 2. | Interleukin-2 Inhibitors Pipeline Report Executive Summary |
| 3. | Interleukin-2 Inhibitors Pipeline: Overview |
| 4. | Analytical Perspective In-depth Commercial Assessment |
| 5. | Interleukin-2 Inhibitors Clinical Trial Therapeutics |
| 6. | Interleukin-2 Inhibitors Pipeline: Late-Stage Products (Pre-registration) |
| 7. | Interleukin-2 Inhibitors Pipeline: Late-Stage Products (Phase III) |
| 8. | Interleukin-2 Inhibitors Pipeline: Mid-Stage Products (Phase II) |
| 9. | Interleukin-2 Inhibitors Pipeline: Early-Stage Products (Phase I) |
| 10. | Interleukin-2 Inhibitors Pipeline Therapeutics Assessment |
| 11. | Inactive Products in the Interleukin-2 Inhibitors Pipeline |
| 12. | Company-University Collaborations (Licensing/Partnering) Analysis |
| 13. | Key Companies |
| 14. | Key Products in the Interleukin-2 Inhibitors Pipeline |
| 15. | Unmet Needs |
| 16. | Market Drivers and Barriers |
| 17. | Future Perspectives and Conclusion |
| 18. | Analyst Views |
| 19. | Appendix |
For further information on the interleukin-2 inhibitors pipeline therapeutics, reach out @ Interleukin-2 Inhibitors Therapeutics
Related Reports
Interleukin-2 Inhibitors Market Size, Target Population, Competitive Landscape & Market Forecast - 2034 report deliver an in-depth understanding of the market trends, market drivers, market barriers, and key IL-2 companies, including Mural Oncology, Corvus Pharmaceuticals, Philogen, Nektar, Cue Biopharma, Krystal Biotech, ILTOO Pharma, Innovent, Regeneron Pharmaceuticals, R2T Biopharma (XEME Biopharma), AstraZeneca, GI Innovation, Merck, Synthekine, BioNTech, Medicenna Therapeutics, Ascendis Pharma, Sanofi, Synthorx, Cugene, AbbVie, Deka Biosciences, Dragonfly Therapeutics, Werewolf Therapeutics, Xilio Therapeutics, Bioniz Therapeutics, Equillium, Asher Bio, Anaveon, Aulos Bioscience, Selecxine, ProBio, Hoffmann-La Roche, TILT Biotherapeutics, among others.
Interleukin-2 Inhibitors Competitive Landscape
Interleukin-2 Inhibitors Market Insights, Competitive Landscape, and Market Forecast – 2032 report provides comprehensive insights about the pipeline landscape, pipeline drug profiles, including clinical and non-clinical stage products, and the key IL-2 inhibitors companies, including Merck, Synthekine, BioNTech, Medicenna Therapeutics, Ascendis Pharma, Sanofi, Synthorx, Cugene, AbbVie, Deka Biosciences, Dragonfly Therapeutics, Werewolf Therapeutics, Xilio Therapeutics, among others.
Interleukin-4 Inhibitors Pipeline
Interleukin-4 Inhibitors Pipeline Insight – 2025 report provides comprehensive insights about the pipeline landscape, pipeline drug profiles, including clinical and non-clinical stage products, and the key IL-4 inhibitors companies, including Regeneron, Sanofi, ASLAN Pharmaceuticals, Akeso Biopharma, AstraZeneca, TaiwanJ Pharmaceuticals, among others.
Interleukin-6 Inhibitors Pipeline
Interleukin-6 Inhibitors Pipeline Insight – 2025 report provides comprehensive insights about the pipeline landscape, pipeline drug profiles, including clinical and non-clinical stage products, and the key IL-6 inhibitors companies, including Novo Nordisk, EUSA Pharma, Celgene Corporation, R-Pharm, Emphycorp, Fountain BioPharma, Hutchison MediPharma, Kodiak Sciences, NeurMedix, Kodiak Sciences, Starton Therapeutics, Peptinov, Biocad, Qyuns Therapeutics, Bio-Thera Solutions, Janssen Biotech, Signpath Pharma, CSL Behring, among others.
Interleukin-8 Receptor Antagonists Pipeline
Interleukin-8 Receptor Antagonists Pipeline Insight – 2025 report provides comprehensive insights about the pipeline landscape, pipeline drug profiles, including clinical and non-clinical stage products, and the key IL-8 receptor antagonist companies, including Dompe Farmaceutici, AstraZeneca, Syntrix Biosystems, Ligand Pharmaceuticals, Aristea Therapeutics, Corvus Pharmaceuticals, among others.
DelveInsight’s Pharma Competitive Intelligence Service: Through its CI solutions, DelveInsight provides its clients with real-time and actionable intelligence on their competitors and markets of interest to keep them stay ahead of the competition by providing insights into the latest therapeutic area-specific/indication-specific market trends, in emerging drugs, and competitive strategies. These services are tailored to the specific needs of each client and are delivered through a combination of reports, dashboards, and interactive presentations, enabling clients to make informed decisions, mitigate risks, and identify opportunities for growth and expansion.
Other Business Pharmaceutical Consulting Services
Healthcare Conference Coverage
Discover how a mid-pharma client gained a level of confidence in their soon-to-be partner for manufacturing their therapeutics by downloading our Due Diligence Case Study
About DelveInsight
DelveInsight is a leading Business Consultant, and Market Research firm focused exclusively on life sciences. It supports pharma companies by providing comprehensive end-to-end solutions to improve their performance.

Contact Us Shruti Thakur info@delveinsight.com +14699457679 www.delveinsight.com
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.
